Me hurry
a calorimeter is a device used to measure how much heat energy is taken in or given...

Chemistry, 25.12.2019 15:31 sofiisabella10
Me hurry
a calorimeter is a device used to measure how much heat energy is taken in or given off in a reaction. suppose after five trials of a chemical reaction, 200 kj of heat was taken in on average. what conclusion can be made about the reaction? a. it is endothermic
.b. it releases heat energy.
c. it is a combustion reaction.
d. it creates more than one product.

Answers: 3


Other questions on the subject: Chemistry

Chemistry, 22.06.2019 12:00, WinterStrikesBack
Solutions of sodium carbonate and silver nitrate react to form solid silver carbonate and a solution of sodium nitrate. a solution containing 3.50 g of sodium carbonate is mixed with one containing 5.00 g of silver nitrate. how many grams of sodium carbonate, silver nitrate, silver carbonate, and sodium nitrate are present after the reaction is complete?
Answers: 2


Chemistry, 22.06.2019 16:30, montanolumpuy
How many moles of sulfuric acid (h2so4) are needed to react completely with 6.8 moles of lithium hydroxide (lioh)? 2lioh + h2so4 → li2so4 + 2h2o a. 3.4 mol h2so4b. 6.8 mol h2so4 c. 10.2 mol h2so4 d. 13.6 mol h2so4
Answers: 3

Chemistry, 22.06.2019 19:50, VoidedAngel
When the mercury level in a barometer decreases that atmospheric pressure has
Answers: 3
You know the right answer?
Questions in other subjects:

Health, 28.08.2019 11:10

Business, 28.08.2019 11:10


History, 28.08.2019 11:10


Mathematics, 28.08.2019 11:10

Social Studies, 28.08.2019 11:10

Chemistry, 28.08.2019 11:10


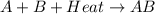
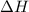 = +ve for an endothermic reaction.
= +ve for an endothermic reaction.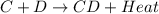 is an exothermic reaction.
is an exothermic reaction. 

