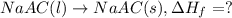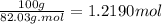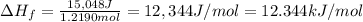Chemistry, 18.10.2019 12:00 christi1175
Acalorimeter contains 500 g of water at 25°c. you place a hand warmer containing 100 g of liquid sodium acetate (naac) inside the calorimeter. when the sodium acetate finishes crystallizing, the temperature of the water inside the calorimeter is 32.2°c. the specific heat of water is
4.18 j/g-°c. what is the enthalpy of fusion (δhf) of the sodium acetate? show your work.

Answers: 2


Other questions on the subject: Chemistry

Chemistry, 22.06.2019 01:00, deaishaajennings123
What is the equilibrium constant of aa+bb=cc+dd
Answers: 1

Chemistry, 22.06.2019 07:00, shradhwaip2426
Achemist wants to extract copper metal from copper chloride solution. the chemist places 0.50 grams of aluminum foil in a solution containing 0.75 grams of copper (ii) chloride. a single replacement reaction takes place. (ii) chloride. a single replacement reaction takes place. which statement explains the maximum amount of copper that the chemist can extract using this reaction? a) approximately 0.36 grams, because copper (ii) chloride acts as a limiting reactant b) approximately 1.8 grams, because copper (ii) chloride acts as a limiting reactant c) approximately 0.36 grams, because aluminum acts as a limiting reactant d) approximately 1.8 grams, because aluminum acts as a limiting reactant
Answers: 3

Chemistry, 22.06.2019 10:00, micahwilkerson9495
Select all of the methods through which a drug can enter your body. injection swallowing inhalation absorption
Answers: 2

Chemistry, 22.06.2019 17:40, aguilarjose
If 3 moles of a compound use 24 j of energy in a reaction, what is the a hreaction in j/mol?
Answers: 1
You know the right answer?
Acalorimeter contains 500 g of water at 25°c. you place a hand warmer containing 100 g of liquid sod...
Questions in other subjects:

Mathematics, 22.06.2019 10:00

Advanced Placement (AP), 22.06.2019 10:00

Chemistry, 22.06.2019 10:00

History, 22.06.2019 10:00


History, 22.06.2019 10:00

French, 22.06.2019 10:00

Spanish, 22.06.2019 10:00


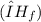 of the sodium acetate is 12.344 kJ/mol.
of the sodium acetate is 12.344 kJ/mol.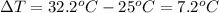
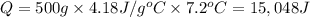
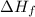 is the heat energy required to melt the one mole of substance.
is the heat energy required to melt the one mole of substance.