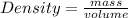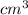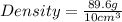Chemistry, 13.01.2020 23:31 charnaetoney13
Daniel has a sample of pure copper. its mass is 89.6 grams (g), and its volume is 10 cubic centimeters (cm3). what’s the density of the sample?
0.11 g/cm3
8.96 g/cm3
11.1 g/cm3
896 g/cm3

Answers: 1


Other questions on the subject: Chemistry

Chemistry, 21.06.2019 14:00, eddsworldfrantic
3. if the dartboard below is used to model an atom, which subatomic particles would be located at z?
Answers: 2

Chemistry, 22.06.2019 13:50, kelonmazon2492
Read the chemical equation. 2c2h2 + 5o2 → 4co2 + 2h2o which of the following statements would be correct if one mole of c2h2 was used in this reaction? one mole of oxygen was used in this reaction. five moles of oxygen were used in this reaction. four moles of carbon dioxide were produced from this reaction. two moles of carbon dioxide were produced from this reaction.
Answers: 3

Chemistry, 22.06.2019 20:30, sydneip6174
We are hoping to create 5.72 grams of glucose. the plant was given 4.75 liters of co2 and 2.81 g of h20. which reactant was the limiting reagent? how much excess mass did we have of the other reactant?
Answers: 2
You know the right answer?
Daniel has a sample of pure copper. its mass is 89.6 grams (g), and its volume is 10 cubic centimete...
Questions in other subjects:



Mathematics, 08.10.2020 14:01


Mathematics, 08.10.2020 14:01


History, 08.10.2020 14:01