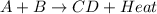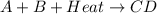Chemistry, 04.02.2020 06:06 adstone814
When the products of a reaction are hotter than the reactants: the reaction is exothermic. the reaction is endothermic. the reactants lost internal energy. the change in enthalpy is positive.

Answers: 1


Other questions on the subject: Chemistry

Chemistry, 21.06.2019 20:30, nxusasmangaliso8780
Which sentence best describes the formation of igneous rock? a- lava on the surface dries up and makes arock b_melted rocks cools and forms crystals c_rocks under tremendous heat and pressure d_magma is melted rock underground
Answers: 1

Chemistry, 21.06.2019 23:00, LarryJoeseph
Why are the trends and exceptions to the trends in ionization energy observed?
Answers: 1

Chemistry, 22.06.2019 10:30, kdenormandie3122
Geothermal energy for industrial use is available almost anywhere. a. true b. false
Answers: 2

Chemistry, 22.06.2019 12:00, kayla32213
Under normal conditions, describe how increasing the temperatures effects the solubility of a typical salt
Answers: 1
You know the right answer?
When the products of a reaction are hotter than the reactants: the reaction is exothermic. the reac...
Questions in other subjects:



Mathematics, 17.05.2021 01:10

Arts, 17.05.2021 01:10

Mathematics, 17.05.2021 01:10



Social Studies, 17.05.2021 01:10