
D: when sodium sulfide dissociates in water, the sulfur ion reacts with water as follows: s-2 + h2o → oh- + sh- which of the following statements is true? na2s is a base because it ionizes to release oh-. na2s is an acid because it is a proton donor. na2s is a base because it increases the hydroxide concentration. none of these

Answers: 3


Other questions on the subject: Chemistry


Chemistry, 22.06.2019 09:00, tashaunalewis4786
This chart lists four kinds of polymers and their sources. what can be known about all four polymers, despite their differences? they come from living things. they share ionic carbon bonds. they are at least 100 monomers long. they are made of repeating subunits.
Answers: 1

Chemistry, 22.06.2019 17:40, adantrujillo1234
Areaction in which products can react to re-form reactants is
Answers: 1
You know the right answer?
D: when sodium sulfide dissociates in water, the sulfur ion reacts with water as follows: s-2 + h2...
Questions in other subjects:



Spanish, 06.01.2020 19:31






Spanish, 06.01.2020 19:31

Biology, 06.01.2020 19:31

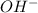 ) upon dissociation in water. This means that a base increases the hydroxide ion concentration in a solution.
) upon dissociation in water. This means that a base increases the hydroxide ion concentration in a solution. 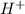 ) upon dissociation in water. This means that an acid increases the hydrogen ion concentration in a solution.
) upon dissociation in water. This means that an acid increases the hydrogen ion concentration in a solution.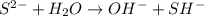
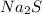 ) dissociates to give hydroxide ions.
) dissociates to give hydroxide ions. 

