
Chemistry, 03.02.2020 13:59 krystinayagel013
Assuming 100% dissociation, calculate the freezing point and boiling point of a 2.5 m sncl4 (aq)

Answers: 2


Other questions on the subject: Chemistry

Chemistry, 22.06.2019 00:30, thatonestudent2271
If 3.00 g of titanium metal is reacted with 6.00 g of chlorine gas, cl2, to form 7.7 g of titanium (iv) chloride in a combination reaction, what is the percent yield of the product?
Answers: 1

Chemistry, 22.06.2019 10:00, shayneseaton
The tendency of water molecules to stick together is referred to as a) adhesion b) polarity c) cohesion d) transpiration e) evaporation
Answers: 1

You know the right answer?
Assuming 100% dissociation, calculate the freezing point and boiling point of a 2.5 m sncl4 (aq)...
Questions in other subjects:

History, 09.10.2019 20:30


Physics, 09.10.2019 20:30

Mathematics, 09.10.2019 20:30



History, 09.10.2019 20:30



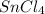 = 2.5
= 2.5
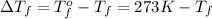
 = freezing point constant= 1.86Kkg/mol
= freezing point constant= 1.86Kkg/mol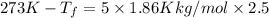
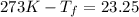

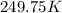 .
.

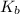 =boiling point constant= 0.512 Kkg/mol
=boiling point constant= 0.512 Kkg/mol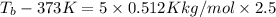
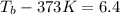
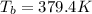
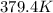 .
.

