
Chemistry, 24.08.2019 19:00 gymnastlyfe123
In the reaction between bromine and sodium, a bromine atom gains an electron. what ion is formed? is the bromine oxidized. or is it reduced?

Answers: 2


Other questions on the subject: Chemistry

Chemistry, 21.06.2019 13:00, velazquez2974
Covalent bonds are formed between metals and boiling points true or false
Answers: 2

Chemistry, 22.06.2019 00:30, lasagnafoe
Used the balanced equation 2h2+ o2 - -> 2h2o. if you have 7.2 grams of o2 , how many grams of h2o can you produce ?
Answers: 2

Chemistry, 22.06.2019 16:00, ghadeeraljelawy
How does blood clotting prevent the entry of pathogens through cuts and wounds? answer asap,, this is due tomorrow. will mark as brainliest or whatever you call it : )
Answers: 2

Chemistry, 22.06.2019 19:20, choiboiqg5755
The equation picture below shows which type of nuclear reaction u 235 + n x e 134 + sr 100 + 2n
Answers: 1
You know the right answer?
In the reaction between bromine and sodium, a bromine atom gains an electron. what ion is formed? i...
Questions in other subjects:

English, 05.02.2020 21:49


Mathematics, 05.02.2020 21:49

Social Studies, 05.02.2020 21:49




Mathematics, 05.02.2020 21:49

Mathematics, 05.02.2020 21:49

Mathematics, 05.02.2020 21:49

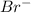 , It is reduced.
, It is reduced.![[Na]=1s^22s^22p^63s^1](/tpl/images/0194/4378/4ee3b.png)
![[Na^+]=1s^22s^22p^63s^0](/tpl/images/0194/4378/79ec1.png)
![[Br]:35:[Ar]3d^{10}4s^24p^5](/tpl/images/0194/4378/c1073.png)
![[Br^-]:36:[Ar]3d^{10}4s^24p^6](/tpl/images/0194/4378/49247.png)



