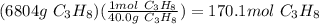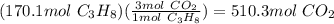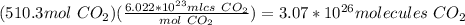Chemistry, 02.09.2019 07:00 kaitlynhess
Propane is used as a fuel in most gas grills to cook food on during the warm summer months. when the propane is burned the following reaction takes place:
c3h8(l) + 5o2(g) = 3co2(g) + 4h20(g)
a standard propane tank contains 6804 g of propane. determine how many molecules of carbon dioxide gas are released into the atmosphere with an entire tank of propane is burned. in your answer be sure to: describe the type of chemical reaction the propane undergoes, calculate the number of moles of propane used in the reaction, explain the mole ratio between propane and carbon dioxide in this reaction, calculate the number of moles of carbon dioxide produced, and calculate the number of molecules of carbon dioxide produced.

Answers: 1


Other questions on the subject: Chemistry

Chemistry, 21.06.2019 22:30, tot92
Imagine that you’re getting ready to move to a new city. when people move, they are influenced by push factors and pull factors, and you have many reasons for your move. which of the following factors is an example of a pull factor? a. wanting to move because you’ve found a great new school somewhere new b. needing to move because there are not enough resources in your old hometown c. being forced to move because your old home is gone d. having to move because there are no jobs in your current hometown
Answers: 1

Chemistry, 22.06.2019 15:00, hockeykid7583
Which are forms of frozen water? check all that apply. dew frost hail rain sleet
Answers: 2

Chemistry, 22.06.2019 19:00, elizabethajih99
Sum of brother and sisters age is 26. four times the brothers age is subtracted from three times the sisters age, the difference is 8. what are the ages of the brother and sister?
Answers: 1
You know the right answer?
Propane is used as a fuel in most gas grills to cook food on during the warm summer months. when the...
Questions in other subjects:

Mathematics, 23.11.2019 17:31

English, 23.11.2019 17:31


Mathematics, 23.11.2019 17:31

Mathematics, 23.11.2019 17:31



English, 23.11.2019 17:31

Business, 23.11.2019 17:31