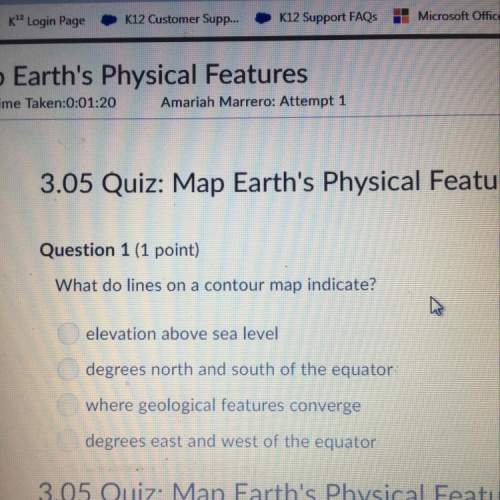
Chemistry, 05.02.2020 02:01 zakarycrane9576
Which of the following solutes will lower the freezing point of water the most?
a) the molecular compound sucrose (c₁₂h₂₂22o₁₁)
b) the iconic compound magnesium sulfate (mgso₄4)
c)the iconic lithium chloride (lici)
d)the iconic compound calcium fluoride(caf₂2)

Answers: 2


Other questions on the subject: Chemistry

Chemistry, 22.06.2019 03:10, emilyplays474
The peak wavelength for the blackbody curve of a star is in the uv range. assuming the radiation from this star can reach earth, would you be able to see it?
Answers: 2

Chemistry, 22.06.2019 08:30, jalst6084
7. using your knowledge of colligative properties explain whether sodium chloride or calcium chloride would be a more effective substance to melt the ice on a slick sidewalk. use 3 – 4 sentences in your explanation. 8. when a 2.5 mol of sugar (c12h22o11) are added to a certain amount of water the boiling point is raised by 1 celsius degree. if 2.5 mol of aluminum nitrate is added to the same amount of water, by how much will the boiling point be changed? show all calculations leading to your answer or use 3 – 4 sentences to explain your answer. 9. if 5.40 kcal of heat is added to 1.00 kg of water at 100⁰c, how much steam at 100⁰c is produced? show all calculations leading to an answer. 10. the freezing of water at 0⁰c can be represented as follows: h2o (l) ↔ h2o(s) the density of liquid water is 1.00 g/cm3. the density of ice is 0.92 g/cm3. in 3 – 4 sentences explain why applying pressure causes ice to melt.
Answers: 1

Chemistry, 22.06.2019 09:00, bibhu42kumarp7o4ss
At 300 mm hg, a gas has a volume of 380 l, what is the volume at standard pressure
Answers: 1

Chemistry, 22.06.2019 12:00, hannah2757
Why are people not able to skip a dive to the deepest part of the ocean
Answers: 1
You know the right answer?
Which of the following solutes will lower the freezing point of water the most?
a) the...
a) the...
Questions in other subjects:


Mathematics, 01.12.2020 08:40

English, 01.12.2020 08:40

Mathematics, 01.12.2020 08:40

Chemistry, 01.12.2020 08:40

Mathematics, 01.12.2020 08:40


Mathematics, 01.12.2020 08:40

Arts, 01.12.2020 08:40

Mathematics, 01.12.2020 08:40

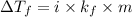
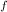 = change in freezing point
= change in freezing point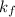 = freezing point constant
= freezing point constant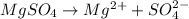 Thus i= 2
Thus i= 2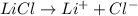 , thus i=2.
, thus i=2.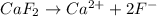 , thus i=3.
, thus i=3.