
Chemistry, 17.10.2019 04:30 Jacobolobo7
Consider this combination reaction:
2mg(s)+o2(g)→2mgo(s) δh=−1204 kj
what is the enthalpy for the decomposition of 1 mole of mgo(s) into mg(s) and o2(g)?
consider this combination reaction:
what is the enthalpy for the decomposition of 1 mole of into and ?
-1204 kj/mol
602 kj/mol
1204 kj/mol
-602 kj/mol

Answers: 1


Other questions on the subject: Chemistry

Chemistry, 22.06.2019 05:00, adrian128383
What forms when chemical reactions combine pollution with sunlight?
Answers: 1

Chemistry, 22.06.2019 10:00, melissa9882
A50.0g sample of liquid water at 0.0 c ends up as ice at -20.0 c. how much energy is involved in this change?
Answers: 1

Chemistry, 22.06.2019 19:00, ecolifesfsu1263
What is the compound name for the formula [ru(en)2cl2]2+ and [co(en)cl2br]-
Answers: 1

Chemistry, 23.06.2019 00:10, Rubendelarosa1529
Covalent compounds: mastery test select the correct answer what is formed when atoms join together with a covalent bond? a. an ion b. a molecule c. a neutral atom d. a noble gas
Answers: 3
You know the right answer?
Consider this combination reaction:
2mg(s)+o2(g)→2mgo(s) δh=−1204 kj
what is the enthal...
2mg(s)+o2(g)→2mgo(s) δh=−1204 kj
what is the enthal...
Questions in other subjects:










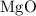 is
is 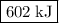 .
.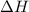 of an overall reaction is obtained by adding the enthalpy change for each individual step reaction involved to obtain the overall reaction.
of an overall reaction is obtained by adding the enthalpy change for each individual step reaction involved to obtain the overall reaction.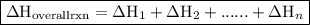
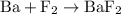
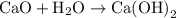
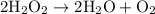
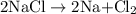
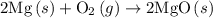
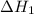 is
is 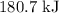 .
.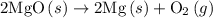
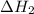 .
.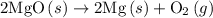 ......(2)
......(2)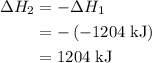
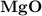 dissociates to give two moles of
dissociates to give two moles of 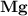 and one mole of
and one mole of 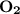 and therefore the enthalpy for the decomposition of one mole of is as follows:
and therefore the enthalpy for the decomposition of one mole of is as follows: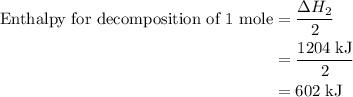
 .
.

