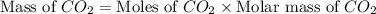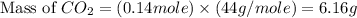You carefully weigh out 14.00 g of caco3 powder and add it to 56.70 g of hcl solution. you notice bubbles as a reaction takes place. you then weigh the resulting solution and find that it has a mass of 64.96 g . the relevant equation is
caco3(s)+2hcl(aq)→h2o(l)+co2(g)+cac l2(aq)
assuming no other reactions take place, what mass of co2 was produced in this reaction?

Answers: 1


Other questions on the subject: Chemistry

Chemistry, 22.06.2019 04:00, miamassimino
The rules of engagement (roe) working group is often used to (select all that apply.)
Answers: 2

Chemistry, 22.06.2019 06:00, mapoohdoll
How much would the freezing point of water decrease if 4 mol of sugar were added to 1 kg of water(k=1.86 c/mol/kg for water and i=1 for sugar
Answers: 1

Chemistry, 22.06.2019 08:30, melikefood01
Sally is making a model of a magnesium atom with an atomic mass number of 24 for her chemistry class. she has foam balls for the protons, neutrons, and electrons. she has added 6 neutrons to her model so far. how many more neutrons does she need to add to complete her neutral atom of magnesium?
Answers: 1

Chemistry, 22.06.2019 09:00, miller5452
Acrystal that absorvd water from air is (blank)a. aqueousb. homogenousc. hygroscopicd. efflorescent
Answers: 1
You know the right answer?
You carefully weigh out 14.00 g of caco3 powder and add it to 56.70 g of hcl solution. you notice bu...
Questions in other subjects:

English, 09.12.2020 16:30



English, 09.12.2020 16:30


Mathematics, 09.12.2020 16:30

Social Studies, 09.12.2020 16:30

Mathematics, 09.12.2020 16:30


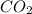 produced will be, 6.16 grams.
produced will be, 6.16 grams.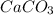 = 14 g
= 14 g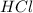 = 56.70 g
= 56.70 g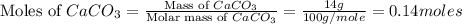
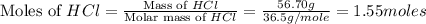
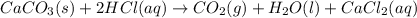
 moles of
moles of