
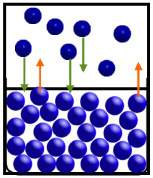
In the diagram below, particles of the substance are moving from the liquid phase to the gas phase at the same rate as they move from the gas phase to the liquid phase.
mc003-1.jpg
the gas and liquid are
~ equilibrium.
~ a high vapor pressure.
~ a low vapor pressure.
~ zero vapor pressure.

Answers: 1


Other questions on the subject: Chemistry



Chemistry, 22.06.2019 14:30, Priskittles
What state of matter is ice a. liquid b. element c. solid d. gas
Answers: 1

Chemistry, 22.06.2019 19:00, nayashuntel
How many liters of ethylene glycol antifreeze (c2h6o2), with a density of 1.100 g/l, would you add to your car radiator containing 15.0 kg of water if you needed to protect your engine to - 21.5°c? for water, kf = 1.86°c m -1.
Answers: 1
You know the right answer?
In the diagram below, particles of the substance are moving from the liquid phase to the gas phase a...
Questions in other subjects:













