
Chemistry, 06.10.2019 00:30 twistedhyperboles
The first step in the reaction of alka-seltzer with stomach acid consists of one mole of sodium bicarbonate (nahco3) reacting with one mole of hydrochloric acid (hcl) to produce one mole of carbonic acid (h2co3), and one mole of sodium chloride (nacl). using this chemical stoichiometry, determine the number of moles of carbonic acid that can be produced from 5 mol of nahco3 and 8 mol of hcl.

Answers: 1


Other questions on the subject: Chemistry

Chemistry, 23.06.2019 00:10, graceception
In as 1°, 2°, 3°, or 4°. be to . : °b: °c: °d: ° : °b: °c: °d: ° : °b: °c: °d: °e: °f: °g: °h: ° : °b: °c: °d: °e: °f: °g: °h: °i: °
Answers: 3

Chemistry, 23.06.2019 00:30, kylee65
What are the advantages of using the metric system? designed as a decimal system making conversions simpler more accurate system of measurement has prefixes that correspond to an amount to use with all base units used by the entire scientific community
Answers: 2

Chemistry, 23.06.2019 01:00, skatelife8974
What is the chemical name of the compound ti2o3?
Answers: 2

You know the right answer?
The first step in the reaction of alka-seltzer with stomach acid consists of one mole of sodium bica...
Questions in other subjects:




Mathematics, 28.01.2020 07:31

English, 28.01.2020 07:31

English, 28.01.2020 07:31





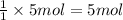 of HCl
of HCl


