
Chemistry, 14.12.2019 12:31 mackenzie27717
Use vsepr theory to predict bond angles in the following covalently bonded molecules. explain your predictions.
a. methane
b. ammonia
c. water

Answers: 2


Other questions on the subject: Chemistry

Chemistry, 22.06.2019 00:30, jamesnaquan132
What is the most stable monatomic ion formed from nitrogen
Answers: 2

Chemistry, 22.06.2019 03:30, asianaenaeh
Select the correct answer. when carbon dioxide dissolves in water, it sometimes reacts with water to form carbonic acid as in this balanced equation: co2 + h2o → h2co3. if 495 milliliters of carbon dioxide at 25°c and 101.3 kilopascals reacts with excess water, what is the theoretical yield of carbonic acid? use the periodic table and the ideal gas resource a. 0.889 g b. 1.10g c. 1.27g d. 2.02g what's the answer! quick!
Answers: 1


Chemistry, 22.06.2019 08:00, mariamakonteh31
Will give ! what are the advantages and disadvantages of nuclear power? check all that apply. one advantage of nuclear energy is that it does not produce carbon dioxide emissions. storage of nuclear waste is a short-term problem associated with nuclear energy. the problem with uranium mining is that a large quantity of uranium must be extracted to meet energy needs because the energy release from uranium fission is so low. safe operation of a nuclear power plant can be jeopardized by a human mistake.
Answers: 1
You know the right answer?
Use vsepr theory to predict bond angles in the following covalently bonded molecules. explain your p...
Questions in other subjects:

Mathematics, 17.10.2019 23:40



Chemistry, 17.10.2019 23:40




Biology, 17.10.2019 23:40

Mathematics, 17.10.2019 23:40

![Formula used :{\text{Number of electrons}} =\frac{1}{2}[V+N-C+A]](/tpl/images/0418/6428/ffc49.png)
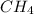
![Formula used :{\text{Number of electrons}} =\frac{1}{2}[4+4-0+0]=4](/tpl/images/0418/6428/2d0c5.png)
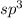 and geometry of the molecule will be tetrahedral.The bond angle for terahedral geometry is 109.5°.
and geometry of the molecule will be tetrahedral.The bond angle for terahedral geometry is 109.5°.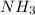
![Formula used :{\text{Number of electrons}} =\frac{1}{2}[5+3-0+0]=4](/tpl/images/0418/6428/13f6a.png)
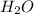
![Formula used :{\text{Number of electrons}} =\frac{1}{2}[6+2-0+0]=4](/tpl/images/0418/6428/1d091.png)



