
Chemistry, 01.01.2020 15:31 kelsotay623
Convert 1.75 × 1024 atoms of carbon to moles of carbon.

Answers: 2


Other questions on the subject: Chemistry

Chemistry, 22.06.2019 13:30, kassandrarosario1115
How many protons, electrons, and neutrons are in each of the following isotopes? a. zirconium-90 b. palladium-108 c. bromine-81 d. antimony-123
Answers: 1


Chemistry, 23.06.2019 12:50, almaga1979orfvwo
Complete the paragraph to describe the characteristics of a borane molecule (bh3). the lewis structure and table of electronegativities are given. the bond polarities in bh3 are , the molecular shape is , and the molecule is .
Answers: 2

Chemistry, 23.06.2019 15:00, RegencySlayer5304
Charlene puts together two isosceles triangles so that they share a base, creating a kite. the legs of the triangles are 10 inches and 17 inches, respectively. if the length of the base for both triangles is 16 inches long, what is the length of the kite’s other diagonal? 6 inches inches inches 21 inchesanswer is d on e2020edit: it's geometry not chemistry, sorry.
Answers: 3
You know the right answer?
Convert 1.75 × 1024 atoms of carbon to moles of carbon....
Questions in other subjects:






History, 01.04.2020 21:57

Biology, 01.04.2020 21:57



Mathematics, 01.04.2020 21:57


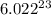 number of atoms are contained in 1 mole of an element.
number of atoms are contained in 1 mole of an element. 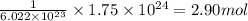 of carbon.
of carbon.

