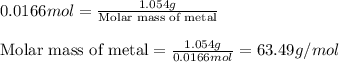Answers: 2


Other questions on the subject: Chemistry

Chemistry, 21.06.2019 13:50, dootdootkazoot
Aluminum–lithium (al-li) alloys have been developed by the aircraft industry to reduce the weight and improve the performance of its aircraft. a commercial aircraft skin material having a density of 2.47 g/cm3 is desired. compute the concentration of li (in wt%) that is required.
Answers: 3

Chemistry, 21.06.2019 17:00, jmanrules200
What is the empirical formula of vanadium 1 oxide given that 20.38 grams of vandium combines with oxygen to form 23.58 grams of the oxide
Answers: 1


Chemistry, 23.06.2019 03:30, uniqueray33
The molar mass of nickel(ni) is 58.7 g/mol. how many moles are in an 88 gram sample of nickel?
Answers: 1
You know the right answer?
When 1.187 g of a metallic oxide is reduced with excess hydrogen 1.054 g of the metal is produced. w...
Questions in other subjects:



Mathematics, 11.10.2020 14:01

History, 11.10.2020 14:01

Geography, 11.10.2020 14:01


Mathematics, 11.10.2020 14:01


English, 11.10.2020 14:01

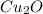
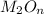
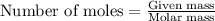 .......(1)
.......(1)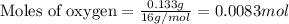
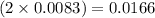 moles
moles