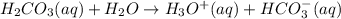Chemistry, 24.09.2019 07:30 Carlosruelas5409
The reaction h2co3 h2o< -> h3o hco3– takes place in water. what happens to the equilibrium when the pressure is increased? (1 point)it favors formation of reactants. it favors formation of products. it does not change. it is conserved.

Answers: 2


Other questions on the subject: Chemistry

Chemistry, 21.06.2019 14:10, carsonjohnsonn
Can smoke be transformed into liquid or used as energy or both?
Answers: 2

Chemistry, 21.06.2019 23:30, mgavyn1052
Write a paragraph that provides examples of each stage of volcanic activity, a description of the volcano, and facts about each stage.
Answers: 1

Chemistry, 22.06.2019 07:00, haydjanggg6578
The boiling point of propanoic acid is higher than that of 1-butanol because: propanoic acid has a higher molecular weight than 1-butanol. propanoic acid is more soluble in water than 1-butanol. propanoic acid is a better hydrogen bond donor than 1-butanol. propanoic acid forms hydrogen bonded dimers and 1-butanol does not. 1-butanol forms hydrogen bonded dimers and propanoic acid does not.
Answers: 2

Chemistry, 22.06.2019 10:30, mv603177
Rocks, as they are compressed, begin forming mountains above the earth's surface when two continental plates converge. the continental crust increases in depth as the mountains grow above. the himalayan mountains formed at a convergent plate boundary in this manner. the rocks are smashed together causing them to due to the intense heat and pressure from the colliding plates and eventually forming rock. a) melt; igneous b) layer; sedimentary c) recrystallize; metamorphic d) melt into the earth's interior; metamorphic
Answers: 1
You know the right answer?
The reaction h2co3 h2o< -> h3o hco3– takes place in water. what happens to the equilibrium wh...
Questions in other subjects:



Mathematics, 02.10.2019 11:20

Business, 02.10.2019 11:20

Mathematics, 02.10.2019 11:20

English, 02.10.2019 11:20


Mathematics, 02.10.2019 11:20


English, 02.10.2019 11:20