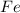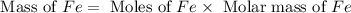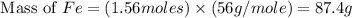Chemistry, 24.11.2019 12:31 briannagisellegarcia
Fe2o3 + 2al -> al2o3 + 2fe
calculate the mass of iron metal (in grams) that can be prepared from 150 grams of aluminum and 250 grams of iron(iii) oxide.
!

Answers: 3


Other questions on the subject: Chemistry



Chemistry, 22.06.2019 06:30, Pizzapegasus1
Three cards with holes are arranged in a straight line. a light is shined through the first card’s hole and travels through all three cards. what does this tell you about light rays? a) that light is reflected b) that light is refractive c) that light travels in a straight line d) that light does not travel in a straight line
Answers: 1

Chemistry, 22.06.2019 13:30, kassandrarosario1115
How many protons, electrons, and neutrons are in each of the following isotopes? a. zirconium-90 b. palladium-108 c. bromine-81 d. antimony-123
Answers: 1
You know the right answer?
Fe2o3 + 2al -> al2o3 + 2fe
calculate the mass of iron metal (in grams) that can be p...
calculate the mass of iron metal (in grams) that can be p...
Questions in other subjects:

Mathematics, 25.06.2019 06:30

English, 25.06.2019 06:30

Biology, 25.06.2019 06:30

Advanced Placement (AP), 25.06.2019 06:30

Mathematics, 25.06.2019 06:30

History, 25.06.2019 06:30



Biology, 25.06.2019 06:30

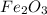 = 250 g
= 250 g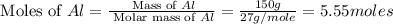
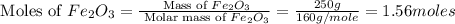
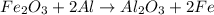
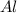 react with 1 mole of
react with 1 mole of 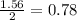 moles of
moles of