
I NEED HELP LIKE RN
Question 1 (1 point)
Answer to question #1.
Question 1 options:
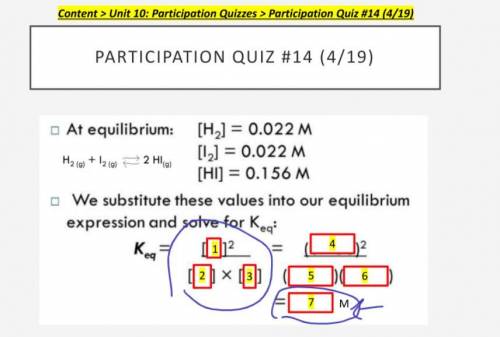
H2
I2
HI
Question 2 (1 point)
Answer to question #2.
Question 2 options:
H2
I2
HI
Question 3 (1 point)
Answer to question #3.
Question 3 options:
H2
I2
HI
Question 4 (1 point)
Answer to question #4.
Question 4 options:
0.022 M
0.156 M
Question 5 (1 point)
Answer to question #5.
Question 5 options:
0.022 M
0.156 M
Question 6 (1 point)
Answer to question #6.
Question 6 options:
0.022 M
0.156 M
Question 7 (1 point)
Answer to question #7.
Question 7 options:
0.003 M
1.11 M
7.09 M
50.3 M

Answers: 3


Other questions on the subject: Chemistry

Chemistry, 21.06.2019 18:10, hunterthompson2
Which is true of transition metals when moving from left to right on the periodic table? the d sublevels are not filled across the period. the cation radii become larger across the period. atomic radii increase slightly and then start to decrease. atomic radii decrease slightly and then start to increase. o
Answers: 2

Chemistry, 21.06.2019 22:30, kiki197701
Agas at 155 kpa and standard temperature has an initial volume of 1.00 l. the pressure of the gas rises to 500 kpa as the temperature also rises to 135°c. what is the new volume? 2.16 l 0.463 l 0.207 l 4.82 l
Answers: 3

Chemistry, 22.06.2019 06:00, VamPL
Oxidation-reduction reactions (often called "redox" for short) are reactions that involve the transfer of electrons from one species to another. oxidation states, or oxidation numbers, allow chemists to keep track of these electron transfers. in general, one element will lose electrons (oxidation), with the result that it will increase in oxidation number, and another element will gain electrons (reduction), thereby decreasing in oxidation number. the species that is oxidized is called the reducing agent or reductant. the species that is reduced is called the oxidizing agent or oxidant. to sum up: oxidation = increase in oxidation state = loss of electrons = reducing agent reduction = decrease in oxidation state = gain of electrons = oxidizing agent part a which element is oxidized in this reaction? fe2o3+3co→2fe+3co2 enter the elemental symbol. view available hint(s) is oxidized part b which element is reduced in this reaction? 2hcl+2kmno4+3h2c2o4→6co2+2mno2+2kcl +4h2o enter the elemental symbol. view available hint(s) is reduced
Answers: 1

Chemistry, 22.06.2019 13:10, bartonamber4042
Select the correct answer a modure consists of glucose and water. what is the percent composition of glucose in the mixture if it contains 1.3 moles of glucose (cho total mass of the mature is 276 grams? ) and the a 1775
Answers: 1
You know the right answer?
I NEED HELP LIKE RN
Question 1 (1 point)
Answer to question #1.
Question 1 o...
Answer to question #1.
Question 1 o...
Questions in other subjects:


Mathematics, 28.06.2019 12:50


English, 28.06.2019 12:50






Geography, 28.06.2019 12:50




