
Chemistry, 19.04.2021 21:00 student679
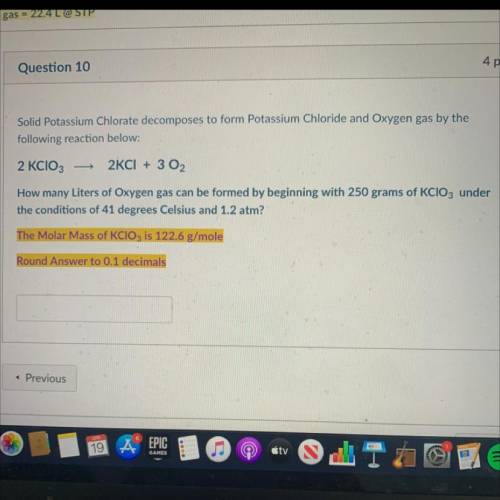
Solid Potassium Chlorate decomposes to form Potassium Chloride and Oxygen gas by the
following reaction below:
2 KCIO3
2KCI + 3 02
How many Liters of Oxygen gas can be formed by beginning with 250 grams of KCIO3 under
the conditions of 41 degrees Celsius and 1.2 atm?
The Molar Mass of KClO3 is 122.6 g/mole
Round Answer to 0.1 decimals

Answers: 3


Other questions on the subject: Chemistry

Chemistry, 22.06.2019 00:30, catdog5225
Drive down any three characteristic of modern periodic table
Answers: 1

Chemistry, 22.06.2019 05:00, Angelanova69134
Frictional forces acting on an object are often converted into energy, which causes the temperature of the object to rise slightly.
Answers: 2

Chemistry, 22.06.2019 10:30, angemango3423
What is the empirical formula of c6h18o3? ch3o c2h5o c2h6o c2h5o5
Answers: 1

You know the right answer?
Solid Potassium Chlorate decomposes to form Potassium Chloride and Oxygen gas by the
following reac...
Questions in other subjects:


Mathematics, 08.03.2021 04:50

Chemistry, 08.03.2021 04:50

Biology, 08.03.2021 04:50



Mathematics, 08.03.2021 04:50


Mathematics, 08.03.2021 04:50

Mathematics, 08.03.2021 04:50



