
Chemistry, 19.04.2021 21:00 TheMixingToad
*Urgent*
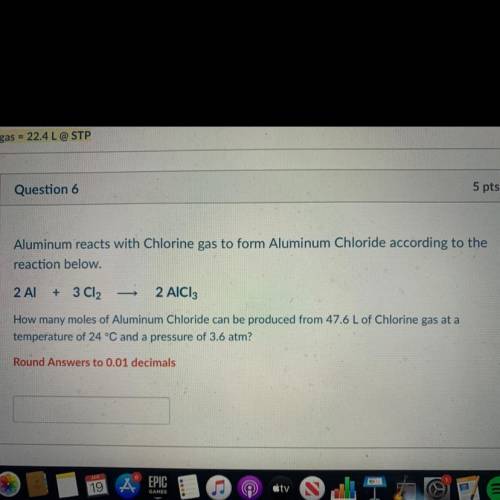
Aluminum reacts with Chlorine gas to form Aluminum Chloride according to the
reaction below.
2 Al
+
3 Cl2
2 AICI:
How many moles of Aluminum Chloride can be produced from 47.6 L of Chlorine gas at a
temperature of 24 °C and a pressure of 3.6 atm?
Round Answers to 0.01 decimals

Answers: 3


Other questions on the subject: Chemistry

Chemistry, 22.06.2019 14:00, luisaareli6298
Calculate the energy required to ionize a hydrogen atom to an excited state where the electron is initially in the n = 5 energy level. report your answer in kilojoules
Answers: 1


Chemistry, 22.06.2019 22:00, jespinozagarcia805
In order to complete this lab. you will need to be familiar with some common chemistry terms. complete the chemical change puzzle and list the relevant terms and their meaning below a. rectant b. product c. supernate
Answers: 3

Chemistry, 23.06.2019 00:00, bryn2433
Predict the relative bond lengths of the three carbon-oxygen bonds in the carbonate ion (co2−3). what would you expect the charge to be on each oxygen? match the words in the left column to the appropriate blanks in the sentences on the right. make certain each sentence is complete before submitting your answer.
Answers: 3
You know the right answer?
*Urgent*
Aluminum reacts with Chlorine gas to form Aluminum Chloride according to the
reactio...
reactio...
Questions in other subjects:



History, 21.08.2019 18:00

Biology, 21.08.2019 18:00


Mathematics, 21.08.2019 18:00


Mathematics, 21.08.2019 18:00


Computers and Technology, 21.08.2019 18:00



