
Chemistry, 19.04.2021 19:40 supasavb99
A 6.50-gram piece of aluminum reacts with an excess of oxygen. Use the balanced equation below to determine how many grams of aluminum oxide is formed during this reaction.
A.662.7 grams of Al2O3
B.24.6 grams of Al2O3
C.12.3 grams of Al2O3
D.6.1 grams of Al2O3
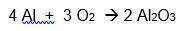

Answers: 1


Other questions on the subject: Chemistry

Chemistry, 22.06.2019 07:00, haydjanggg6578
The boiling point of propanoic acid is higher than that of 1-butanol because: propanoic acid has a higher molecular weight than 1-butanol. propanoic acid is more soluble in water than 1-butanol. propanoic acid is a better hydrogen bond donor than 1-butanol. propanoic acid forms hydrogen bonded dimers and 1-butanol does not. 1-butanol forms hydrogen bonded dimers and propanoic acid does not.
Answers: 2

Chemistry, 22.06.2019 12:30, AnastasiaJauregui
Which of the following describes a compound? (hint: carbon and oxygen bo a. a piece of pure carbon, containing only carbon atoms b. oxygen gas surrounding a solid piece of carbon c. a substance made of two oxygen atoms for each carbon atom carbon and oxygen atoms mixed without being bonded together
Answers: 1

Chemistry, 22.06.2019 18:00, LuvieAnn1886
How is energy related to the change of state represented by the model? atoms gain energy as a solid changes to a liquid. atoms gain energy as a solid changes to a gas. atoms lose energy as a solid changes to a liquid. atoms lose energy as a solid changes to a gas.
Answers: 3

Chemistry, 22.06.2019 22:50, kanerobertrosss2213
At the current rate, a graph of carbon dioxide produced by fossil fuels over time would slope upward slope downward be horizontal be vertical
Answers: 3
You know the right answer?
A 6.50-gram piece of aluminum reacts with an excess of oxygen. Use the balanced equation below to de...
Questions in other subjects:




Mathematics, 30.12.2021 07:20

Social Studies, 30.12.2021 07:20

Biology, 30.12.2021 07:20

Chemistry, 30.12.2021 07:20

Biology, 30.12.2021 07:20





