
Chemistry, 19.04.2021 07:10 irelandcrawford5469
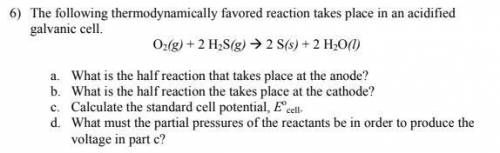
The following thermodynamically favored reaction takes place in an acidified
galvanic cell.
O2(g) + 2 H2S(g) 2 S(s) + 2 H2O(l)
a. What is the half reaction that takes place at the anode?
b. What is the half reaction the takes place at the cathode?
c. Calculate the standard cell potential, Eo
cell.
d. What must the partial pressures of the reactants be in order to produce the
voltage in part c?

Answers: 3


Other questions on the subject: Chemistry


Chemistry, 22.06.2019 14:30, emilymartinez75
Need ! asap will mark 10 pts using the room temperature line (orange line) and your periodic table, make lists that identify the state of matter (gas, liquid, or solid) in which each element you plotted exists at room temperature. explain your answers
Answers: 1


Chemistry, 22.06.2019 15:30, alaf05160
Two metal blocks that have slightly different temperatures are placed next to one another. after five minutes, they both have lower but equal temperatures. according to the law of conservation of energy, what most likelyhappened? energy was created inside the blocks. energy was destroyed inside the blocks. energy was absorbed into the blocks from outside the system. energy was transferred from the warmer block to the cooler block.
Answers: 2
You know the right answer?
The following thermodynamically favored reaction takes place in an acidified
galvanic cell.
Questions in other subjects:


Biology, 26.08.2019 01:30

Mathematics, 26.08.2019 01:30

Physics, 26.08.2019 01:30


History, 26.08.2019 01:30

Mathematics, 26.08.2019 01:30

Advanced Placement (AP), 26.08.2019 01:30

Mathematics, 26.08.2019 01:30

History, 26.08.2019 01:30



