
Chemistry, 18.04.2021 01:00 raquelle66
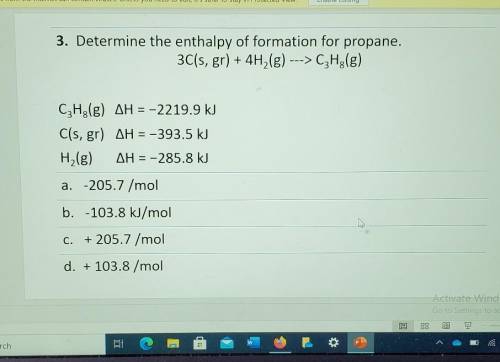
3. Determine the enthalpy of formation for propane. 3C(s, gr) + 4H2(g) ---> C3H2(g) CzH3(g) AH = -2219.9 kJ C(s, gr) AH = -393.5 kJ H,(g) AH = -285.8 kJ a. -205.7 /mol b. -103.8 kJ/mol Å C. + 205.7 /mol d. + 103.8 /mol

Answers: 3


Other questions on the subject: Chemistry

Chemistry, 21.06.2019 22:10, leo4687
What does a particular point on a line of a phase diagram represent? o a. the maximum temperature a substance can exist at without bonds breaking b. the pressure created by the kinetic energy of molecules at a particular temperature c. the melting point or boiling point of a substance at a specific pressure d. the conditions in which temperature and pressure have equal effects on a substance
Answers: 2

You know the right answer?
3. Determine the enthalpy of formation for propane. 3C(s, gr) + 4H2(g) ---> C3H2(g) CzH3(g) AH =...
Questions in other subjects:


Mathematics, 20.01.2021 09:50

English, 20.01.2021 09:50

Chemistry, 20.01.2021 09:50

English, 20.01.2021 09:50

Health, 20.01.2021 09:50

Computers and Technology, 20.01.2021 09:50

Mathematics, 20.01.2021 09:50

Mathematics, 20.01.2021 09:50

English, 20.01.2021 09:50



