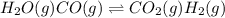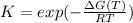Chemistry, 17.04.2021 20:00 andrejr0330jr
For the following exothermic reaction system at equilibrium:
H2O(g) CO(g) CO2(g) H2(g)
Choose the changes that will increase the value of K.
a. Decrease the volume (constant T)
b. Add H2O(g) (constant T)
c. Remove H2(g) (constant T)
d. Add a catalyst (constant T)
e. Add CO2(g) (constant T)
f. Increase the temperature
g. Decrease the temperature

Answers: 2


Other questions on the subject: Chemistry



Chemistry, 22.06.2019 13:50, aesthetickait
How does the motion of particles in a gas change as the gas cools
Answers: 2

Chemistry, 22.06.2019 19:00, elizabethajih99
Sum of brother and sisters age is 26. four times the brothers age is subtracted from three times the sisters age, the difference is 8. what are the ages of the brother and sister?
Answers: 1
You know the right answer?
For the following exothermic reaction system at equilibrium:
H2O(g) CO(g) CO2(g) H2(g)
Choos...
Choos...
Questions in other subjects:




History, 15.01.2021 20:30

Mathematics, 15.01.2021 20:30


Mathematics, 15.01.2021 20:30


Biology, 15.01.2021 20:30