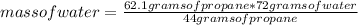Chemistry, 17.04.2021 03:00 ayoismeisalex
How many grams of water, H 2 O , is given o when 62.1 g of propane, C 3 H 8 , burns?

Answers: 3


Other questions on the subject: Chemistry

Chemistry, 21.06.2019 15:30, hannah5143
Determine the empirical formula of a compound containing 40.6 grams of carbon, 5.1 grams of hydrogen, and 54.2 grams of oxygen. in an experiment, the molar mass of the compound was determined to be 118.084 g/mol. what is the molecular formula of the compound? for both questions, show your work or explain how you determined the formulas by giving specific values used in calculations.
Answers: 3

Chemistry, 22.06.2019 04:00, lucasrandall
Asolution contains 225 g of sodium chloride, nacl, dissolved in enough water to make a 0.25 l of solution. what is the molarity of the solution?
Answers: 2

Chemistry, 22.06.2019 07:30, reaperqueen21
Compare and contrast the bohr model and the electron cloud models of the atom.
Answers: 1

Chemistry, 22.06.2019 17:40, aguilarjose
If 3 moles of a compound use 24 j of energy in a reaction, what is the a hreaction in j/mol?
Answers: 1
You know the right answer?
How many grams of water, H 2 O , is given o when 62.1 g of propane, C 3 H 8 , burns?...
Questions in other subjects:

English, 29.10.2020 23:00



Mathematics, 29.10.2020 23:00

Chemistry, 29.10.2020 23:00




Mathematics, 29.10.2020 23:00