
Chemistry, 17.04.2021 02:50 serenitynycole
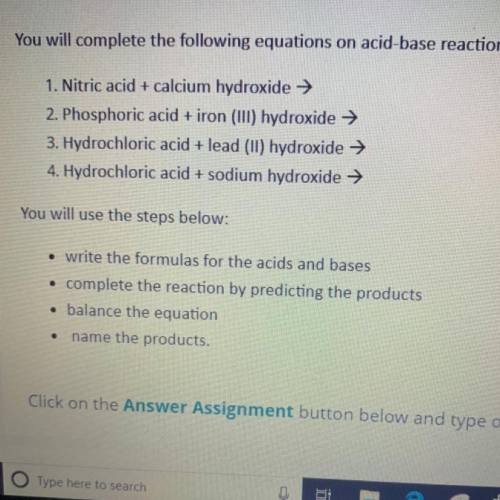
You will complete the following equations on acid-base reactions.
1. Nitric acid + calcium hydroxide →
2. Phosphoric acid + iron (III) hydroxide →
3. Hydrochloric acid + lead (11) hydroxide →
4. Hydrochloric acid + sodium hydroxide →
You will use the steps below:
• write the formulas for the acids and bases
• complete the reaction by predicting the products
• balance the equation
name the products.

Answers: 2


Other questions on the subject: Chemistry


Chemistry, 22.06.2019 11:30, ansuaprajita1506
Voltaic cells produce a positive overall charge. what does this indicate? a. the reaction is likely to be endothermic. b. the reaction is spontaneous. c. the reaction is not likely to occur. d. the reaction is not spontaneous.
Answers: 3

Chemistry, 22.06.2019 12:40, whitethunder05
When 13.3 g koh is dissolved in 102.7 g of water in a coffee-cup calorimeter, the temperature rises from 21.4 °c to 31.53 °c. what is the enthalpy change per gram of koh (j/g) dissolved in the water? * take the density of water as 1.00 g/ml. * assume that the solution has a specific heat capacity of 4.18 j/g*k. enter to 1 decimal place. do not forget the appropriate sign /(+). canvas may auto-delete the (+) sign
Answers: 2
You know the right answer?
You will complete the following equations on acid-base reactions.
1. Nitric acid + calcium hydroxid...
Questions in other subjects:

History, 16.09.2019 04:10

Mathematics, 16.09.2019 04:10


Mathematics, 16.09.2019 04:10


History, 16.09.2019 04:10

Mathematics, 16.09.2019 04:10

Mathematics, 16.09.2019 04:10

Mathematics, 16.09.2019 04:10

Mathematics, 16.09.2019 04:10



