Chemistry, 17.04.2021 01:40 npellot123
2C4H10+13O2-->8CO2+10H2O Using the predicted and balanced equation, How many Liters of CO2 can be produced from 150 grams of C4H10?

Answers: 2


Other questions on the subject: Chemistry

Chemistry, 21.06.2019 15:00, chhimmidemg
How has the scientific community addressed the safety of chemicals? a. chemicals are repeatedly tested, even those that have existed for a long time. b. existing chemicals are tested if they have never been tested before. c. chemicals are tested if they are suspected to have caused a problem. d. only new chemicals are tested.
Answers: 2


Chemistry, 22.06.2019 05:30, mandy9386
You are making a solution of calcium chloride dissolved in water. you add solid, stir, and it dissolves. you add just a spatula tip full, stir, and the solid does not dissolve. how could you describe the solutions before and after adding the spatula tip amount
Answers: 1
You know the right answer?
2C4H10+13O2-->8CO2+10H2O Using the predicted and balanced equation, How many Liters of CO2 can be...
Questions in other subjects:

Mathematics, 02.07.2019 09:00

English, 02.07.2019 09:00


History, 02.07.2019 09:00




Biology, 02.07.2019 09:00


Social Studies, 02.07.2019 09:00

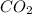 will be produced from 150 grams of
will be produced from 150 grams of 
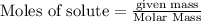


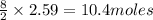 of
of