
Chemistry, 16.04.2021 22:40 daynafish13
Which of the following is the correct set of numbers needed to balance the equation: _C3H8 + _O2→ _CO2+ _H2O
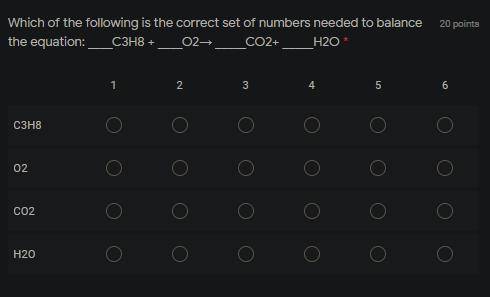

Answers: 2


Other questions on the subject: Chemistry

Chemistry, 21.06.2019 17:10, shafferakr6
There are 6.022 x 10^23 atoms of hg in 1 mole of hg. the number of atoms in 4.5 moles of hg can be found by multiplying 4.5 by 6.022 x 10^23 a. 2.7 x 10^24 b. 27 x 10^23 c. 2.71 x10^24 d. 27.099 x 10^23
Answers: 3


Chemistry, 22.06.2019 11:30, ansuaprajita1506
Voltaic cells produce a positive overall charge. what does this indicate? a. the reaction is likely to be endothermic. b. the reaction is spontaneous. c. the reaction is not likely to occur. d. the reaction is not spontaneous.
Answers: 3

Chemistry, 22.06.2019 12:00, zamariahyou
A5.000 g sample of niso4 h2o decomposed to give 2.755 g of anhydrous niso4. what is the formula of the hydrate? what is the full chemical name for the hydrate? what is the molar mass of the hydrate? niso4•_h2o what is the mass % of water in the hydrate?
Answers: 1
You know the right answer?
Which of the following is the correct set of numbers needed to balance the equation: _C3H8 + _O2→ _C...
Questions in other subjects:

Mathematics, 21.06.2019 14:00

Arts, 21.06.2019 14:00






Social Studies, 21.06.2019 14:00

Mathematics, 21.06.2019 14:00



