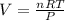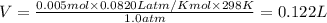Chemistry, 16.04.2021 20:50 elliswilliams1063
You are performing a reaction involving the collection of a gas at SATP. If 5.00 g of magnesium is added to 100.0 cm3 of 0.100 mol dm−3 HCl, determine the volume of hydrogen gas that will be collected at SATP.

Answers: 1


Other questions on the subject: Chemistry




Chemistry, 23.06.2019 01:30, sanchezvazquez0123
In which phase of mitosis do the spindle fibers pull the chromosomes apart to opposite sides of the cell ?
Answers: 1
You know the right answer?
You are performing a reaction involving the collection of a gas at SATP. If 5.00 g of magnesium is a...
Questions in other subjects:

English, 25.10.2019 01:43

History, 25.10.2019 01:43


Mathematics, 25.10.2019 01:43






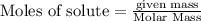
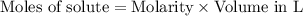
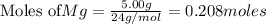
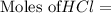
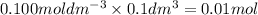
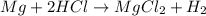
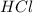 will require=
will require=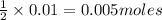 of
of 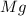
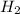
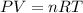
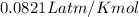
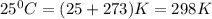 (SATP)
(SATP)