
Chemistry, 16.04.2021 19:10 jaffeisabel
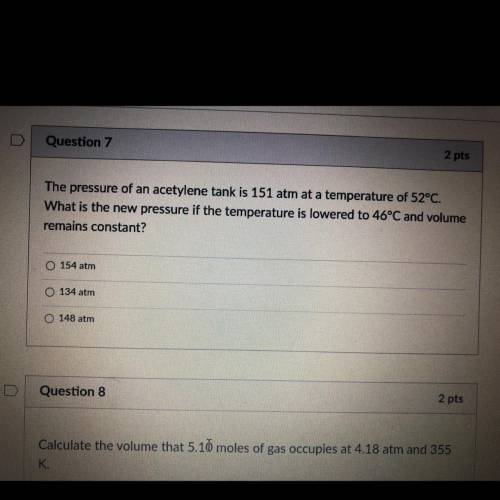
The pressure of an acetylene tank is 151 atm at a temperature of 52°C.
What is the new pressure if the temperature is lowered to 46°C and volume
remains constant?

Answers: 1


Other questions on the subject: Chemistry

Chemistry, 22.06.2019 03:50, Pizzapegasus1
Express the following number in scientific notation. 0.026890 =
Answers: 1

Chemistry, 22.06.2019 12:30, johnsont8377
Avariable that is not being directly tested during an experiment should be changed varied experimented controlled
Answers: 1

Chemistry, 22.06.2019 13:00, naomicervero
Asubstance is a good conductor of electricity which of the following best explains a probable position of the substance in a periodic table
Answers: 3
You know the right answer?
The pressure of an acetylene tank is 151 atm at a temperature of 52°C.
What is the new pressure if...
Questions in other subjects:










English, 06.07.2019 05:30



