Answers: 2


Other questions on the subject: Chemistry

Chemistry, 22.06.2019 07:30, candigirl8847
All cells are made of four types of acids, lipids, proteins, and carbohydrates.
Answers: 1

Chemistry, 22.06.2019 19:30, simihehe
Phosphorous can form an ion called phosphide, which has the formula p3−. this ion can form an ion called phosphide, which has the formula p3−. this ion properties very similar to those of pforms when a phosphorus atom loses three protonsis called a cationcontains 18 electrons
Answers: 2

Chemistry, 23.06.2019 02:30, puppylover72
Calculate the ph at the equivalence point for the titration of a solution containing 150.0 mg of ethylamine (c2h5nh2) with 0.1000 m hcl solution. the volume of the solution at the equivalence point is 250.0 ml. kb forethylamine is 4.7 × 10−4 .
Answers: 2

Chemistry, 23.06.2019 04:00, winterblanco
If you are told to get 100 ml of stock solution to use to prepare smaller size sample for an experiment, which piece of glassware would you use?
Answers: 1
You know the right answer?
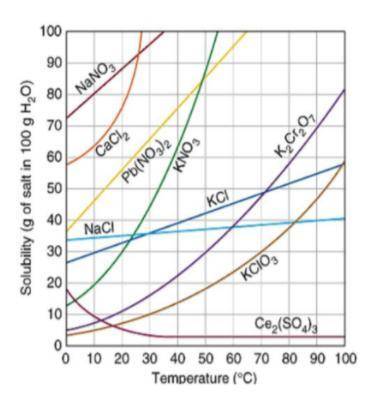
Another student is attempting to make a saturated solution of KCl. The student added 30 g of KCl to...
Questions in other subjects:

History, 03.07.2019 06:00

Mathematics, 03.07.2019 06:00

Mathematics, 03.07.2019 06:00

Mathematics, 03.07.2019 06:00


Mathematics, 03.07.2019 06:00



History, 03.07.2019 06:00

Biology, 03.07.2019 06:00