
Chemistry, 16.04.2021 17:50 donaldwilliams31
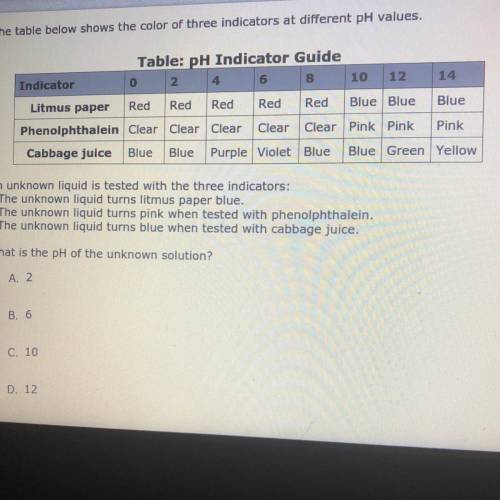
The table below shows the color of three indicators at different pH values.
An unknown liquid is tested with the three indicators:
• The unknown liquid turns litmus paper blue.
• The unknown liquid turns pink when tested with phenolphthalein.
• The unknown liquid turns blue when tested with cabbage juice.
What is the pH of the unknown solution?

Answers: 2


Other questions on the subject: Chemistry

Chemistry, 22.06.2019 04:00, nothingworksoutforme
The image shows a process that releases nuclear energy which statement best identifies the process shown the process must be fusion because energy is released the process must be fusion because of have your nucleus formed a smaller nuclei the process must be fission because a large nucleus breaks into smaller nuclei the process must be fission because neutrons are formed
Answers: 1


Chemistry, 22.06.2019 20:00, aksambo4707
Many free radicals combine to form molecules that do not contain any unpaired electrons. the driving force for the radical–radical combination reaction is the formation of a new electron‑pair bond. consider the chemical equation. n(g)+no(g)⟶nno(g) n(g)+no(g)⟶nno(g) write lewis formulas for the reactant and product species in the chemical equation. include nonbonding electrons. n(g)n(g) select draw rings more erase select draw rings more erase select draw rings more erase n no(g)
Answers: 1
You know the right answer?
The table below shows the color of three indicators at different pH values.
An unknown liquid is te...
Questions in other subjects:












