
Chemistry, 16.04.2021 17:30 SiegeHatake4534
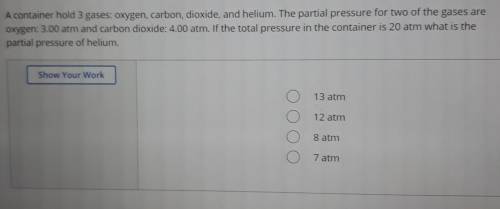
A container hold 3 gases: oxygen, carbon, dioxide, and helium. The partial pressure for two of the gases are oxygen: 3.00 atm and carbon dioxide: 4.00 atm. If the total pressure in the container is 20 atm what is the partial pressure of helium.

Answers: 2


Other questions on the subject: Chemistry

Chemistry, 21.06.2019 18:30, ElizabethF
Calculate the change in entropy if br2(l) is converted to br2(g). s° for br2(l) = 152.2 j/(mol•k) s° for br2(g) = 245.5 j/(mol•k) s° for br(g) = 175.0 j/(mol•k)
Answers: 3

Chemistry, 21.06.2019 21:00, jasminortega2002
Which of the following compounds does not contain molecules? question 2 options: co2 h2 nacl h2o
Answers: 1

Chemistry, 22.06.2019 01:00, kangasc6124
Which type of orbits are found in the principal energy level n = 2 a - s b - s, f c - s, d d - s, p e - s, p, d
Answers: 1

You know the right answer?
A container hold 3 gases: oxygen, carbon, dioxide, and helium. The partial pressure for two of the g...
Questions in other subjects:

Mathematics, 26.06.2019 13:10

Mathematics, 26.06.2019 13:10

Mathematics, 26.06.2019 13:10


Mathematics, 26.06.2019 13:10

Mathematics, 26.06.2019 13:10

Health, 26.06.2019 13:10

English, 26.06.2019 13:10

Business, 26.06.2019 13:10



