Answers: 2


Other questions on the subject: Chemistry

Chemistry, 21.06.2019 22:00, tgraveslaylay2743
Bose-einstein condensation occurs at what temperature?
Answers: 2


Chemistry, 22.06.2019 12:20, jessicasbss6840
Adeuteron, 21h, is the nucleus of a hydrogen isotope and consists of one proton and one neutron. the plasma of deuterons in a nuclear fusion reactor must be heated to about 3.02×108 k . what is the rms speed of the deuterons? express your answer using two significant figures.
Answers: 1
You know the right answer?
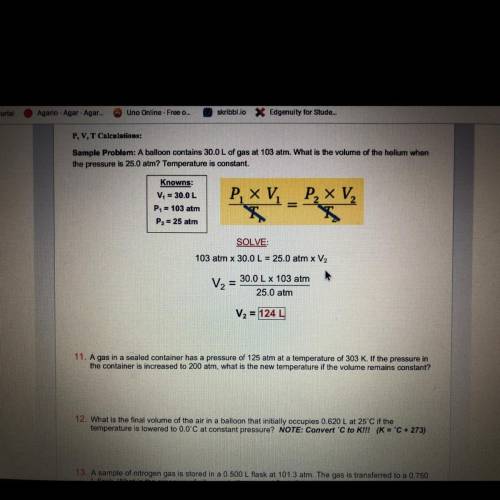
A gas in a sealed container has a pressure of 125 atm at a temperature of 303 K. If the pressure in...
Questions in other subjects:

Mathematics, 13.10.2020 05:01

Mathematics, 13.10.2020 05:01




Mathematics, 13.10.2020 05:01

History, 13.10.2020 05:01


Mathematics, 13.10.2020 05:01