
Chemistry, 16.04.2021 06:30 andrwisawesome0
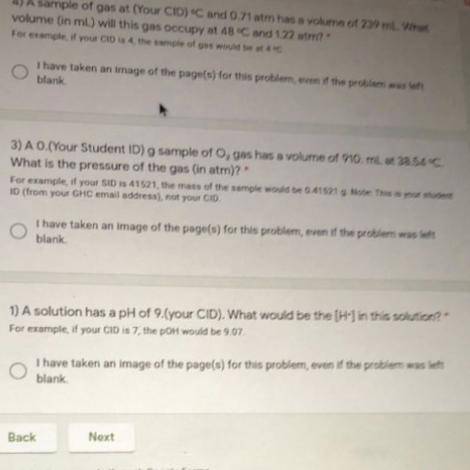
PLEASE HELP PLEASE (my student ID is 36833 so it’s: A 0.36833 g sample of O2 gas has a volume of 910. mL at 38.54 degrees C. What is the pressure of the gas (in atm) ?

Answers: 2


Other questions on the subject: Chemistry


Chemistry, 22.06.2019 12:00, Alexislol7908
From the options provided for each element below, choose the properties that it may have based on its location in the periodic table fluorine (f): highly reactive nonmetal shiny a conductor
Answers: 1

Chemistry, 22.06.2019 18:30, rosie20052019
You open a can of soda at room temperature and hear a hiss. which of the following factors has changed inside the container? a.) atmospheric pressure b.) temperature of gas c.) type of gas d.) amount of gas
Answers: 1

Chemistry, 22.06.2019 20:00, denaemarie02
Nitrogen dioxide decomposes according to the reaction 2 no2(g) ⇌ 2 no(g) + o2(g) where kp = 4.48 × 10−13 at a certain temperature. if 0.70 atm of no2 is added to a container and allowed to come to equilibrium, what are the equilibrium partial pressures of no(g) and o2(g)
Answers: 2
You know the right answer?
PLEASE HELP PLEASE (my student ID is 36833 so it’s: A 0.36833 g sample of O2 gas has a volume of 910...
Questions in other subjects:

History, 24.08.2019 06:20




Biology, 24.08.2019 06:20


Mathematics, 24.08.2019 06:20



Mathematics, 24.08.2019 06:20



