Which of the following is an INCORRECT interpretation of the following reaction?
...

Chemistry, 15.04.2021 21:00 kayranicole1
Which of the following is an INCORRECT interpretation of the following reaction?
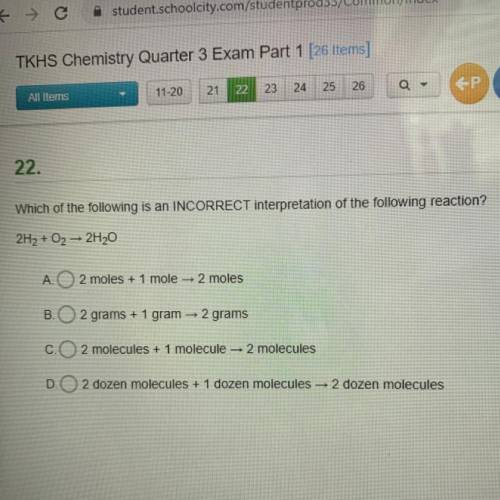

Answers: 2


Other questions on the subject: Chemistry

Chemistry, 21.06.2019 17:30, penny3109
The following reaction shows the products when sulfuric acid and aluminum hydroxide react. al(oh)3 + h2so4 → al2(so4)3 + h2o the table shows the calculated amounts of reactants and products when the reaction was conducted in a laboratory. initial mass and yield sulfuric acid aluminum hydroxide initial amount of reactant 40 g 15 g theoretical yield of water from reactant 14.69 g 10.38 g what is the approximate amount of the leftover reactant? 11.73 g of sulfuric acid 10.33 g of sulfuric acid 11.12 g of aluminum hydroxide 13.67 g of aluminum hydroxide
Answers: 1

Chemistry, 22.06.2019 03:30, fbillinton
In this chemical reaction, 325 grams of barium (ba) react completely. how many moles of lithium (li) are produced?
Answers: 1

Chemistry, 22.06.2019 20:00, AaronEarlMerringer
What is the molar mass of the anhydrous compound? answer using four significant figures. 36.02 g/mol 120.15 g/mol 156.12 g/mol
Answers: 1

Chemistry, 23.06.2019 01:30, heavendl13
How is the solubility of a carbon dioxide gas in water increase?
Answers: 1
You know the right answer?
Questions in other subjects:

Mathematics, 23.02.2021 08:50





Mathematics, 23.02.2021 08:50

Mathematics, 23.02.2021 08:50

Mathematics, 23.02.2021 08:50

Mathematics, 23.02.2021 08:50