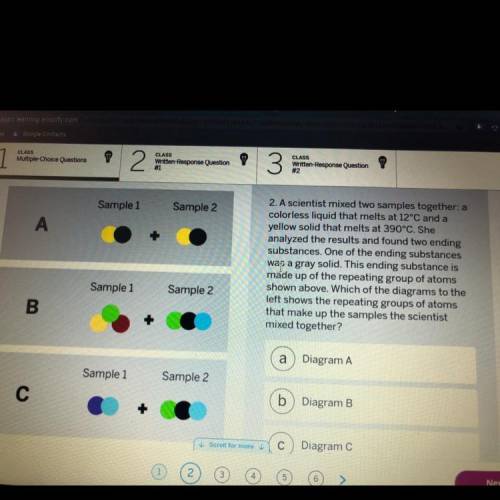
A scientist mixed two samples together: a
colorless liquid that melts at 12°C and a
yellow so...

Chemistry, 15.04.2021 18:10 playaajosh
A scientist mixed two samples together: a
colorless liquid that melts at 12°C and a
yellow solid that melts at 390°С. She
analyzed the results and found two ending
substances. One of the ending substances
was a gray solid. This ending substance is
made up of the repeating group of atoms
shown above. Which of the diagrams to the
left shows the repeating groups of atoms
that make up the samples the scientist
mixed together?

Answers: 2


Other questions on the subject: Chemistry

Chemistry, 22.06.2019 03:50, daniel9299
Consider the reaction: n2(g) + o2(g) ? 2no(g) kc = 0.10 at 2000oc starting with initial concentrations of 0.040 mol/l of n2 and 0.040 mol/l of o2, calculate the equilibrium concentration of no in mol/l how would this be done?
Answers: 3

Chemistry, 22.06.2019 14:50, alexabbarker9781
How are evaporation and sublimation similar? a both involve the formation of a gas. b both release energy to the surroundings. c both take place throughout a solid. d both take place at the surface of a liquid.
Answers: 1

Chemistry, 23.06.2019 04:40, twinchristiansp4xhd2
6) (a) calculate the absorbance of the solution if its concentration is 0.0278 m and its molar extinction coefficient is 35.9 l/(mol cm). the depth of the cell is 5 mm. (b) what is the %t? (7) calculate the absorbance of the solution if the transmitted light intensity is 70% of the initial light beam intensity
Answers: 1
You know the right answer?
Questions in other subjects:



Mathematics, 20.06.2021 14:00

Social Studies, 20.06.2021 14:00



Health, 20.06.2021 14:00

Physics, 20.06.2021 14:00

English, 20.06.2021 14:00




