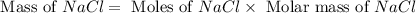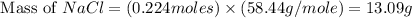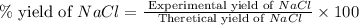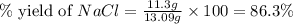Answers: 1


Other questions on the subject: Chemistry

Chemistry, 22.06.2019 02:50, Jerrikasmith28
The conventional equilibrium constant expression (kc) for the system below is: 2icl(s) ⇄ i2(s) + cl2(g) [cl2] ([i2] + [cl2])/2[icl] [i2][cl2]/[icl]2 none of the listed answers are correct [i2][cl2]/2[icl]
Answers: 2

Chemistry, 22.06.2019 04:00, nikkih1225
Electric charge is what ? a. kinetic energy b. radiation c. discovery d. electricity
Answers: 1


Chemistry, 22.06.2019 08:00, wizz4865
Why is the bond angle in a water molecule less than the bond angle of methane? a. the central oxygen atom in water has two lone pairs of electrons, whereas the central carbon atom in methane has no lone pairs. b. the central hydrogen atom in water has one lone pair of electrons, whereas the central carbon atom in methane has two lone pairs. c. the central oxygen atom in water has four lone pairs of electrons, whereas the central carbon atom in methane has only one lone pair. d. the central oxygen atom exerts more repulsive force on surrounding atoms than the central carbon atom in methane does. reset next
Answers: 2
You know the right answer?
When 15 grams of copper (ii) chloride (cucl2) reacts with 20 grams of sodium nitrate (nano3), 11.3 g...
Questions in other subjects:



Mathematics, 20.07.2019 09:30

Mathematics, 20.07.2019 09:30

Mathematics, 20.07.2019 09:30

Health, 20.07.2019 09:30

History, 20.07.2019 09:30

Mathematics, 20.07.2019 09:30

Mathematics, 20.07.2019 09:30

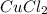 = 15 g
= 15 g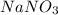 = 20 g
= 20 g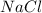 = 58.44 g/mole
= 58.44 g/mole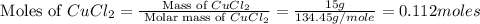
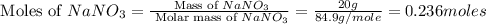
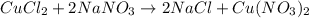
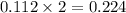 moles of
moles of