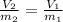Answers: 2


Other questions on the subject: Chemistry

Chemistry, 21.06.2019 14:10, yellowmiki6647
The rock in a lead ore deposit contains 89 % pbs by mass. how many kilograms of the rock must be processed to obtain 1.5 kg of pb?
Answers: 1

Chemistry, 22.06.2019 06:30, yolo123321
The following reaction shows sodium carbonate reacting with calcium hydroxide. na2co3 + ca(oh)2 → naoh + caco3 how many grams of naoh are produced from 20.0 grams of na2co3? (molar mass of na = 22.989 g/mol, c = 12.01 g/mol, o = 15.999 g/mol, ca = 40.078 g/mol, h = 1.008 g/mol) 12.2 grams 15.1 grams 24.4 grams 30.2 grams
Answers: 2

Chemistry, 22.06.2019 07:00, erickamurillo9929
In the cathode ray tube experiment, j. j. thomson passed an electric current through different gases inside a cathode ray tube in the presence of an electric field. in which two ways did this experiment change scientists’ understanding of the atom?
Answers: 2

Chemistry, 22.06.2019 19:00, miguel454545
Avolleyball player hit a ball with a mass of 0.25 kg. the average acceleration of the ball is 15.5 m/s². how much force did the volleyball player apply to the ball? 62.0 n 3.87 n 62.0 m/s² 3.87 m/s²
Answers: 2
You know the right answer?
A 38.2g sample of hydrogen gas, H, is placed into a 2-L soda bottle. IfI keep the pressure and
temp...
Questions in other subjects:





Biology, 16.01.2020 21:31



Biology, 16.01.2020 21:31