
The student wants to test the conductivity of each solution. Prior to carrying out the investigation, the student needs to identify the variables being controlled and the variables being changed between the two solutions. Which identification of the variables is correct?
Options:
The volume of the solution and the concentration of the solution are being changed between the two solutions, but the number of solute particles is being held constant.
The number of solute particles and the concentration of the solution are being changed between the two solutions, but the volume is being held constant.
The number of solute particles is being changed between the two solutions, but the volume and concentration of the solution is being held constant.
The volume of the solution and the number of solute particles are being changed between the two solutions, but the concentration of the solution is being held constant.
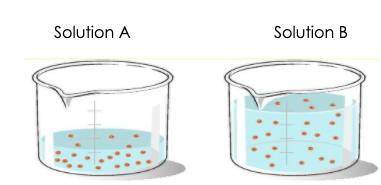

Answers: 3


Other questions on the subject: Chemistry

Chemistry, 22.06.2019 12:10, yootmytoot
Achemistry student needs to standardize a fresh solution of sodium hydroxide. he carefully weighs out of oxalic acid , a diprotic acid that can be purchased inexpensively in high purity, and dissolves it in of distilled water. the student then titrates the oxalic acid solution with his sodium hydroxide solution. when the titration reaches the equivalence point, the student finds he has used of sodium hydroxide solution. calculate the molarity of the student's sodium hydroxide solution. be sure your answer has the correct number of significant digits.
Answers: 1

Chemistry, 22.06.2019 13:00, nadikadiaz1
These questions are based on the attached photo. the experiment is about burning magnesium metal with oxygen. 1. write the balanced chemical equation for the reaction you are performing. 2. calculate the mass of magnesium metal used in each trial. o trial 1: o trial 2: 3. calculate the actual yield of magnesium oxide for each trial. o trial 1: o trial 2: 4. magnesium is the limiting reactant in this experiment. calculate the theoretical yield of mgo for each trial. o trial 1: o trial 2: 5. determine the percent yield of mgo for your experiment for each trial. o trial 1: o trial 2: 6. determine the average percent yield of mgo for the two trials. your company currently uses a process with a similar cost of materials that has an average percent yield of 91 percent. if the average percent yield of this process is higher than that, this could save the company money. what is your recommendation to the company? support your recommendation using your data, calculations, and understanding of stoichiometry gathered from this lab.
Answers: 1

Chemistry, 22.06.2019 13:50, hannahmyung1113
Amap that uses a range of colors and shading to represent the elevation, depth, or landscape of specific features on earth is a/an map.
Answers: 3

Chemistry, 22.06.2019 18:40, johnnysteeler9934
What is one real world example of a colligative property?
Answers: 2
You know the right answer?
The student wants to test the conductivity of each solution. Prior to carrying out the investigation...
Questions in other subjects:

Social Studies, 29.10.2020 16:50







Mathematics, 29.10.2020 16:50





