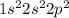Which of the following is true of carbon?
carbon can bond with other carbon atoms, sharing a maximum of eight electrons.
carbon has a very low melting point because c-c bonds are weak.
carbon can form single, double, or triple bonds with another carbon atom.
none of the above.

Answers: 2


Other questions on the subject: Chemistry

Chemistry, 22.06.2019 04:00, clairebear66
What three natural resources are found in the great lakes region
Answers: 2

Chemistry, 22.06.2019 15:00, kamkam5791
Is powdered sports drink ionic or covalent ? 10pts !
Answers: 1

Chemistry, 22.06.2019 22:40, lindseyklewis1p56uvi
Percent ionization for a weak acid (ha) is determined by the following formula: percent ionization=[ha] ionized[ha] initial×100%for strong acids, ionization is nearly complete (100%) at most concentrations. however, for weak acids, the percent ionization changes significantly with concentration. the more diluted the acid is, the greater percent ionization. a certain weak acid, ha, has a ka value of 9.4×10? 7.part acalculate the percent ionization of ha in a 0.10 m solution. part bcalculate the percent ionization of ha in a 0.010 m solution
Answers: 1

You know the right answer?
Which of the following is true of carbon?
carbon can bond with other carbon atoms, sharing a...
carbon can bond with other carbon atoms, sharing a...
Questions in other subjects:


Mathematics, 12.02.2020 22:28


Mathematics, 12.02.2020 22:28



Computers and Technology, 12.02.2020 22:28


History, 12.02.2020 22:28

Mathematics, 12.02.2020 22:28