
Chemistry, 14.04.2021 19:50 tmmackie1748
How much water would I need to add to 700 mL of a 2.7 M KCl solution to make a 1.0 M solution?

Answers: 3


Other questions on the subject: Chemistry

Chemistry, 22.06.2019 12:00, Unknowndragon42
Consider the following reaction at equilibrium. 2co2 (g) 2co (g) + o2 (g) h° = -514 kj le châtelier's principle predicts that the equilibrium partial pressure of co (g) can be maximized by carrying out the reaction a. at high temperature and high pressure b. at high temperature and low pressure c. at low temperature and low pressure d. at low temperature and high pressure e. in the presence of solid carbon
Answers: 2

Chemistry, 22.06.2019 12:30, skaterwolf1317
Which statement is true about this reaction? 14n+1h 15o it is a practical source of energy on earth. it occurs only outside the solar system. its product is heavier than each of its reactants. it shows the critical mass of an element.
Answers: 2

Chemistry, 22.06.2019 14:30, malenacastillo4887
For the reaction shown, find the limiting reactant for each of the following initial amounts of reactants. 4al(s)+3o2(g)→2al2o3(s) a) 1 molal, 1 mol o2 b) 4 molal, 2.6 mol o2 c) 16 molal, 13 mol o2 d) 7.4 molal, 6.5 mol o2
Answers: 3

You know the right answer?
How much water would I need to add to 700 mL of a 2.7 M KCl solution to make a 1.0 M solution?...
Questions in other subjects:


Mathematics, 19.01.2021 23:50

Mathematics, 19.01.2021 23:50

Mathematics, 19.01.2021 23:50


Mathematics, 19.01.2021 23:50

Health, 19.01.2021 23:50


Mathematics, 19.01.2021 23:50

Mathematics, 19.01.2021 23:50

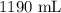
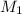 = Initial Concentration of KCl = 2.7 M
= Initial Concentration of KCl = 2.7 M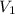 = Volume of KCl = 1 M
= Volume of KCl = 1 M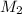 = Final concentration of KCl = 1 M
= Final concentration of KCl = 1 M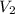 = Amount of water
= Amount of water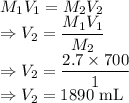
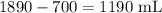 .
.

