
Chemistry, 14.04.2021 18:10 2022mcwhirterbrendan
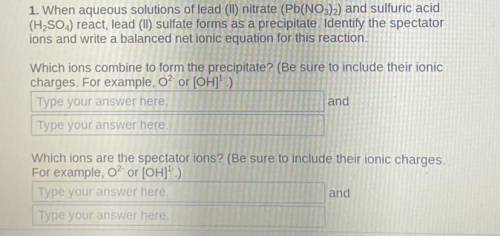
1. When aqueous solutions of lead (II) nitrate (Pb(NO3)2) and sulfuric acid
(H2SO4) react, lead (II) sulfate forms as a precipitate.
Identify the spectator
ions and write a balanced net ionic equation for this reaction.
Which ions combine to form the precipitate? (Be sure to include their ionic
charges. For example, o? or [OH]1- )
Which ions are the spectator ions?

Answers: 3


Other questions on the subject: Chemistry

Chemistry, 21.06.2019 20:40, ellemarshall13
Which of the following pressures is equal to 760 mm hg? 2.0 atm 101.3 pa 101,300 kpa 101,300 pa
Answers: 2

Chemistry, 22.06.2019 12:40, valenzueladomipay09u
How does concentration affect reaction rate
Answers: 2

Chemistry, 22.06.2019 16:30, jrfranckowiak
At 20°c, a sample of h2o liquid and a sample of co2 gas each have the same average kinetic energy. why is one a liquid and the other a gas at this temperature?
Answers: 1
You know the right answer?
1. When aqueous solutions of lead (II) nitrate (Pb(NO3)2) and sulfuric acid
(H2SO4) react, lead (II...
Questions in other subjects:


History, 15.04.2020 17:39




Spanish, 15.04.2020 17:39







