Answers: 1


Other questions on the subject: Chemistry


Chemistry, 22.06.2019 17:10, glitterpanda2468
Calculate the estimated density of each ball. use the formula d = m/v where d is the density, m is the mass, and v is the volume. record your calculations in table a of your student guide. given that the density of water is 1.0 g/cm3, make a prediction about whether each ball will float in water. record your prediction in table a. what is the estimated density of the table tennis ball? record your answer to the nearest hundredth
Answers: 2


Chemistry, 22.06.2019 19:30, Karinaccccc
How might this scientific phenomena be explained? a paper clip floats on water.
Answers: 1
You know the right answer?
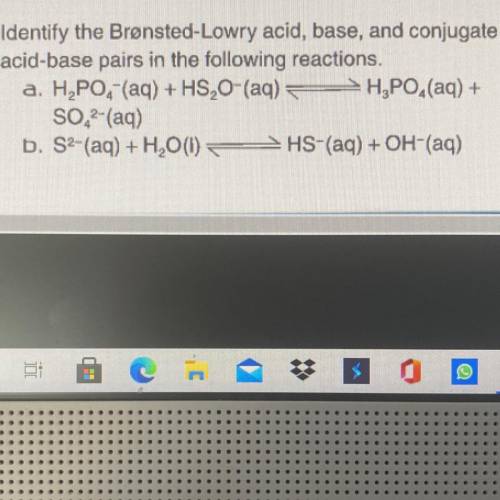
Identify the Bronsted-Lowry Acid, base, and conjugate acid-base pairs in the following reactions. H2...
Questions in other subjects:

Medicine, 14.02.2020 00:58

English, 14.02.2020 00:58

Mathematics, 14.02.2020 00:58