
Chemistry, 27.11.2019 21:31 officialgraciela67
a strong monoprotic acid ionizes in water. if the [h3o+] concentration is 1.56 x 10^-4 m, what else is true?
ph = 10.19
the solution contains more hydronium ions than hydroxide ions.
poh = 1.56 x 10^-4 m
the solution contains equal concentrations of hydroxide and hydronium ions.

Answers: 2


Other questions on the subject: Chemistry

Chemistry, 22.06.2019 05:10, hadellolo8839
How many miles of water are produced if 5.43 mol pbo2 are consumed
Answers: 1

Chemistry, 23.06.2019 00:30, tateandvioletAHS14AY
How many moles of co2 are produced during the complete combustion of 3.6 moles of c2h6
Answers: 1

You know the right answer?
a strong monoprotic acid ionizes in water. if the [h3o+] concentration is 1.56 x 10^-4 m, what else...
Questions in other subjects:


Mathematics, 29.09.2020 16:01

Mathematics, 29.09.2020 16:01

Mathematics, 29.09.2020 16:01

Biology, 29.09.2020 16:01

Mathematics, 29.09.2020 16:01


Mathematics, 29.09.2020 16:01


![pH=-\log [H_3O^+]](/tpl/images/0393/6570/841e8.png)
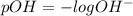
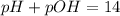
![[H_3O^+]=1.56\times 10^{-4}M](/tpl/images/0393/6570/af221.png)
![pH=-\log [1.56\times 10^{-4}]](/tpl/images/0393/6570/68ee9.png)
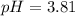
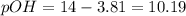
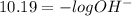
![[{OH^-}]=6.45\times 10^{-11}M](/tpl/images/0393/6570/6f301.png)
![[H^+]](/tpl/images/0393/6570/07acb.png) >
> ![[OH^-]](/tpl/images/0393/6570/b2910.png)


