

Answers: 2


Other questions on the subject: Chemistry

Chemistry, 22.06.2019 06:00, palomaresmitchelle
There are 6.022, 104 atoms of hg in 1 mole of hg the number of atoms in 45 moles of hg can be found by multiplying 4.5 by 6.022, 102 which is the number of atoms in 4.5 moles of hg, correctly written in scientific notation with the correct number of significant figures? 0 21,109 0 21,100 271, 1024 27.099, 100 mark this and retum save and exit submit
Answers: 1


Chemistry, 22.06.2019 09:30, raizagisselle1694
Mike and mitchell decide to have a foot race. they mark off a stretch of 100 yards, and recruit cindy to work the stopwatch. after running the race and looking at the results, cindy declared that mitchell was the fastest. so how did the boys times compare?
Answers: 3

Chemistry, 22.06.2019 12:50, martinez6221
What is the chemical name of the compound na2co3? use the list of polyatomic ions and the periodic table to you answer. a. sodium carbon oxide b. sodium carbonate c. sodium(ll) carbonate d. sodium oxalate
Answers: 1
You know the right answer?
There are 1.806x10^25 molecules of water (h20). how many moles of water does the sample contain?
Questions in other subjects:


English, 29.01.2020 20:45

History, 29.01.2020 20:45


Mathematics, 29.01.2020 20:45

Mathematics, 29.01.2020 20:45


History, 29.01.2020 20:45

Chemistry, 29.01.2020 20:45

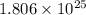
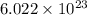 molecules of water present in 1 mole of water.
molecules of water present in 1 mole of water.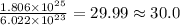 mole of water.
mole of water.


