
Chemistry, 13.04.2021 08:00 Isaacochoa780
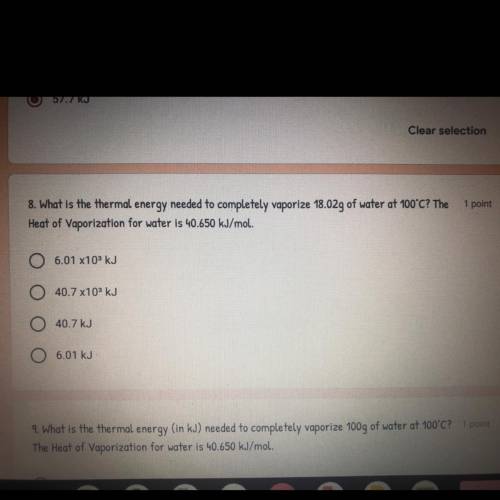
What is the thermal energy needed to vaporize 18.02g of water at 100°C? The Heat of Vaporization for water is 40.650 kJ/mol.

Answers: 2


Other questions on the subject: Chemistry

Chemistry, 22.06.2019 05:00, adrian128383
What forms when chemical reactions combine pollution with sunlight?
Answers: 1



Chemistry, 22.06.2019 18:00, kamjay2006
The human activities in two locations are described below: location a: rampant use of plastic containers location b: excessive use of pesticides and fertilizers which statement is most likely true? location a will have poor air quality because plastic is biodegradable. location a will experience water scarcity because plastic absorbs moisture. the population of honeybees will increase in location b because production of crops will increase. the population of fish in location b will decrease because the water is contaminated.
Answers: 1
You know the right answer?
What is the thermal energy needed to vaporize 18.02g of water at 100°C? The Heat of Vaporization for...
Questions in other subjects:


Mathematics, 04.11.2019 07:31

English, 04.11.2019 07:31


Mathematics, 04.11.2019 07:31


Mathematics, 04.11.2019 07:31

Chemistry, 04.11.2019 07:31

Physics, 04.11.2019 07:31

History, 04.11.2019 07:31




