Chemistry, 13.04.2021 03:10 carlosleblanc26
2Fe(OH)3 Fe2O3+3H2O how many grams of Fe2O3 are produced if 10.7 grams of Fe (OH)3 react in this way

Answers: 2


Other questions on the subject: Chemistry

Chemistry, 21.06.2019 16:30, monithebtslover01
Find the empirical formula of each of the following compounds. given mass or for each element in a sample of the compound 3,611 g ca; 6.389 g c1
Answers: 1

Chemistry, 22.06.2019 00:30, tdowling331
What must happen before a body cell can begin mitotic cell division
Answers: 2

Chemistry, 22.06.2019 20:00, rafaelasoareschagas7
The picture represents the process that produces most of the energy used by living organisms on earth. which process is represented in the picture? a) the magnetic attraction between two hydrogen nuclei. b) the fusion of hydrogen nuclei to produce a helium nucleus in the core of the sun. c) the fission of hydrogen nuclei to produce a helium nucleus in the core of the sun. d) the chemical reaction between hydrogen nuclei to produce a helium nucleus in earth's atmosphere.
Answers: 3

Chemistry, 23.06.2019 01:00, ZaNiyahlove4711
Which of the following is the molecular formula for a simple sugar? a. cooh b. h2o c. oh d. c6h12o6
Answers: 1
You know the right answer?
2Fe(OH)3 Fe2O3+3H2O how many grams of Fe2O3 are produced if 10.7 grams of Fe (OH)3 react in this way...
Questions in other subjects:




Medicine, 06.11.2020 09:00

History, 06.11.2020 09:00

History, 06.11.2020 09:00


Mathematics, 06.11.2020 09:00


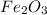 are produced if 10.7 grams of
are produced if 10.7 grams of 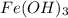 are reacted.
are reacted.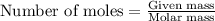 .....(1)
.....(1) 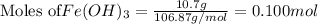
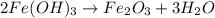
 of
of