
Chemistry, 12.04.2021 21:00 zappygal923
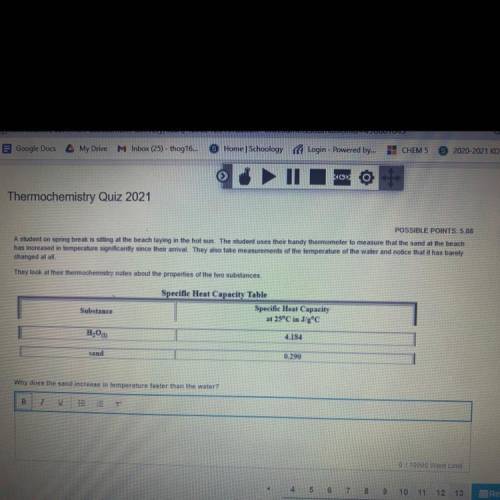
A student on spring break is sitting at the beach laying in the hot sun. The student uses their handy thermometer
to measure that the sand at the beach has increased in temperature significantly since their arrival. They
also take measurements of the temperature of the water and notice that it has barely changed at all.
They look at their thermochemistry notes about the properties of the two substances,
Why does the sand increase in temperature faster than the water?
PLZZZ HELPPP!! ASAP

Answers: 1


Other questions on the subject: Chemistry

Chemistry, 22.06.2019 22:00, jlegrand9098
4.25g sample of solid ammonium nitrate dissolves in 60.0g of water in a coffee-cup calorimeter, the temperature drops from 22.0 c to 16.9 c. assume that the specific heat of the solution is the same as that of pure water. calculate delta(h) (in kj/mol nh4no3) for the solution proces.
Answers: 2

Chemistry, 22.06.2019 23:00, maddyleighanne
Arectangle has a diagonal 20 inches long that forms angles of 60 and 30 with the sides. find the perimeter of the rectangle. for geometry
Answers: 3

Chemistry, 23.06.2019 02:00, FailingstudentXD
Calculate the molarity of each aqueous solution: a. 78.0 ml of 0.240 m naoh diluted to 0.250 l with water b. 38.5 ml of 1.2 m hno3 diluted to 0.130 l with water
Answers: 1

Chemistry, 23.06.2019 04:00, ayoismeisjjjjuan
How many liters of water can be produced from 5.0liters of butane gas at stp, assuming excess oxygen? c4h10(g) + 02(g) → co2 (e) + h2o (g)
Answers: 2
You know the right answer?
A student on spring break is sitting at the beach laying in the hot sun. The student uses their hand...
Questions in other subjects:







History, 22.08.2019 10:30

Mathematics, 22.08.2019 10:30


Mathematics, 22.08.2019 10:30



